Your global partner for clinical contract solutions
Successfully managing all contracts that are required to perform a clinical study
Find the right solution for your specific use case
Are you planning to perform one or more clinical trials and need assistance with your contracts?
We can support you at different stages of the clinical study, in any phase or set-up.

We are Salvius
Salvius supports you in speeding up processes, reducing risks and creating a stable basis for your collaboration with the different stakeholders. We offer you tailored site contracting solutions accommodating your study type and size, and your desired collaboration model.
-
An international team of dedicated lawyers
-
Industry experience
-
Country-specific knowledge
-
Flexible contracting resources
-
Timelines, quality and risk management
- All our contract-experts are ICH-GCP Trained

Our track record
5000+ agreements
We have negotiated over five thousand site agreements

250+ Studies
We were involved in contract management for over two hundred and fifty clinical studies

50+ countries
We have negotiated agreements in more than 50 countries

Indication areas experience
Including oncology, cardiology, neurology and RSV studies

Since 2010
Successfully offering our services since 2010
For an in-depth expertise overview and download in your own language Go to factsheet
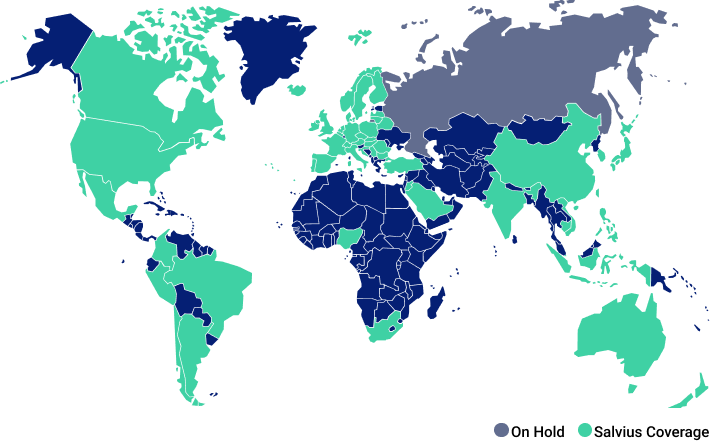
Global Coverage
Salvius is negotiating contracts with sites in nearly every country where clinical research is performed. Our international team covers multiple languages and provides expert country knowledge and experience to support your team.
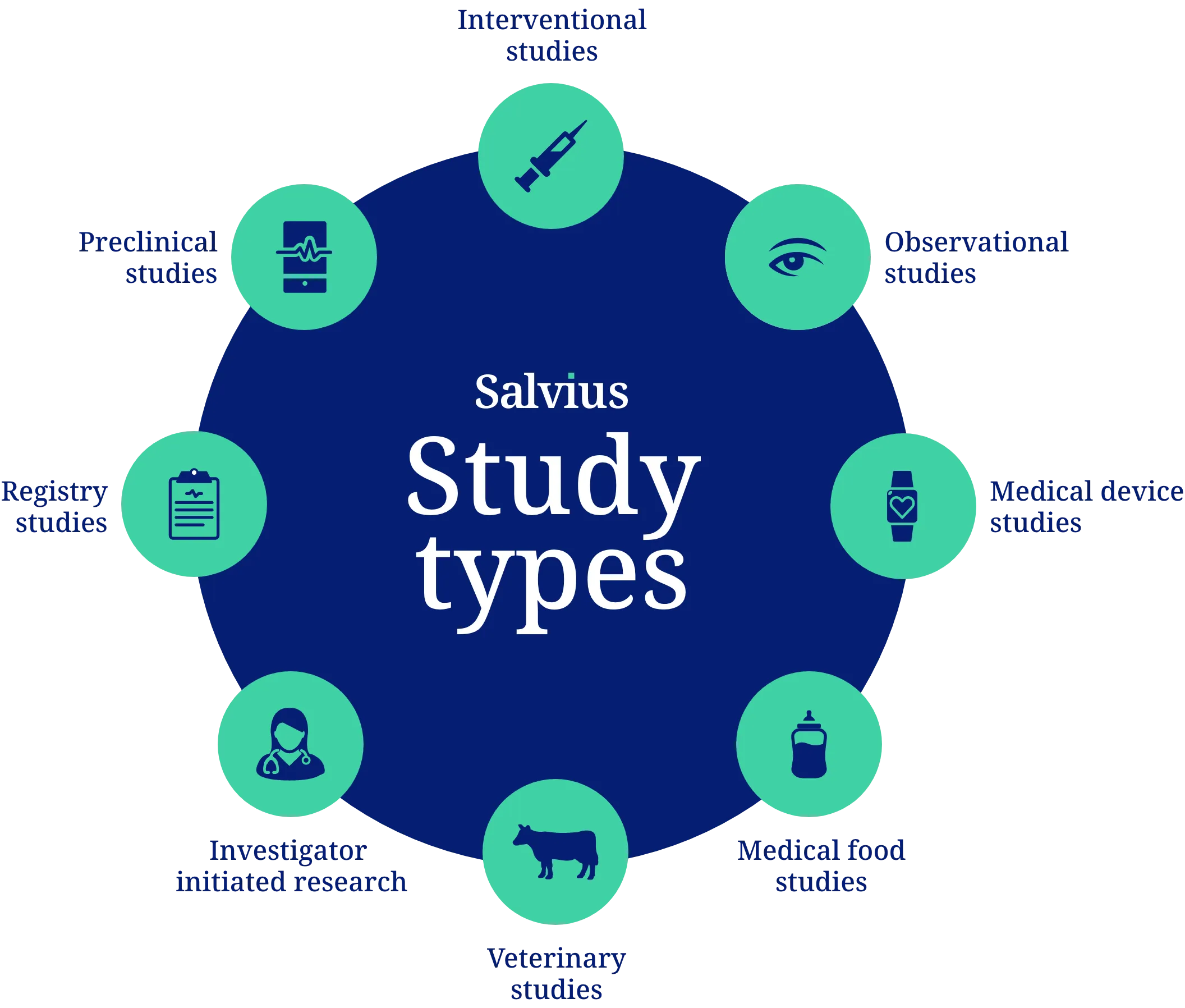
Contract management for all study types
Our clinical contracting services cover a broad range of study types, customizable to different setups for small local studies to large international trials.
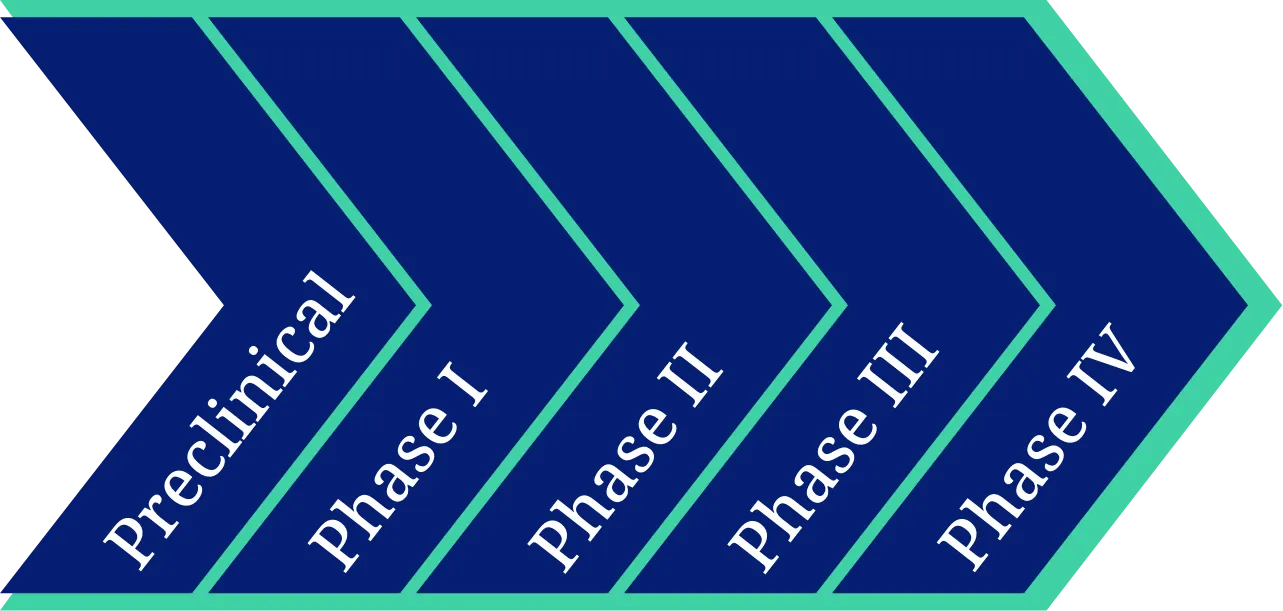
In every product development phase
Salvius offers contract management and legal support for studies in every stage of the product development process. Many of our collaborations start in the earlier phases, which allows us to be aware of our client’s company policies and overall study program contract requirements. Developing a working relationship early on is beneficial in later stages when site and vendor contracting becomes a more important part of the study start-up process, since by then a proper collaboration model has already been established.
Our clients

Our clients include:
- Pharma
- Biotech
- Biopharma
- Medical device
- Registry
- Foundation
- Patient association
- Investigator-Sponsor
- Contract Research Organization